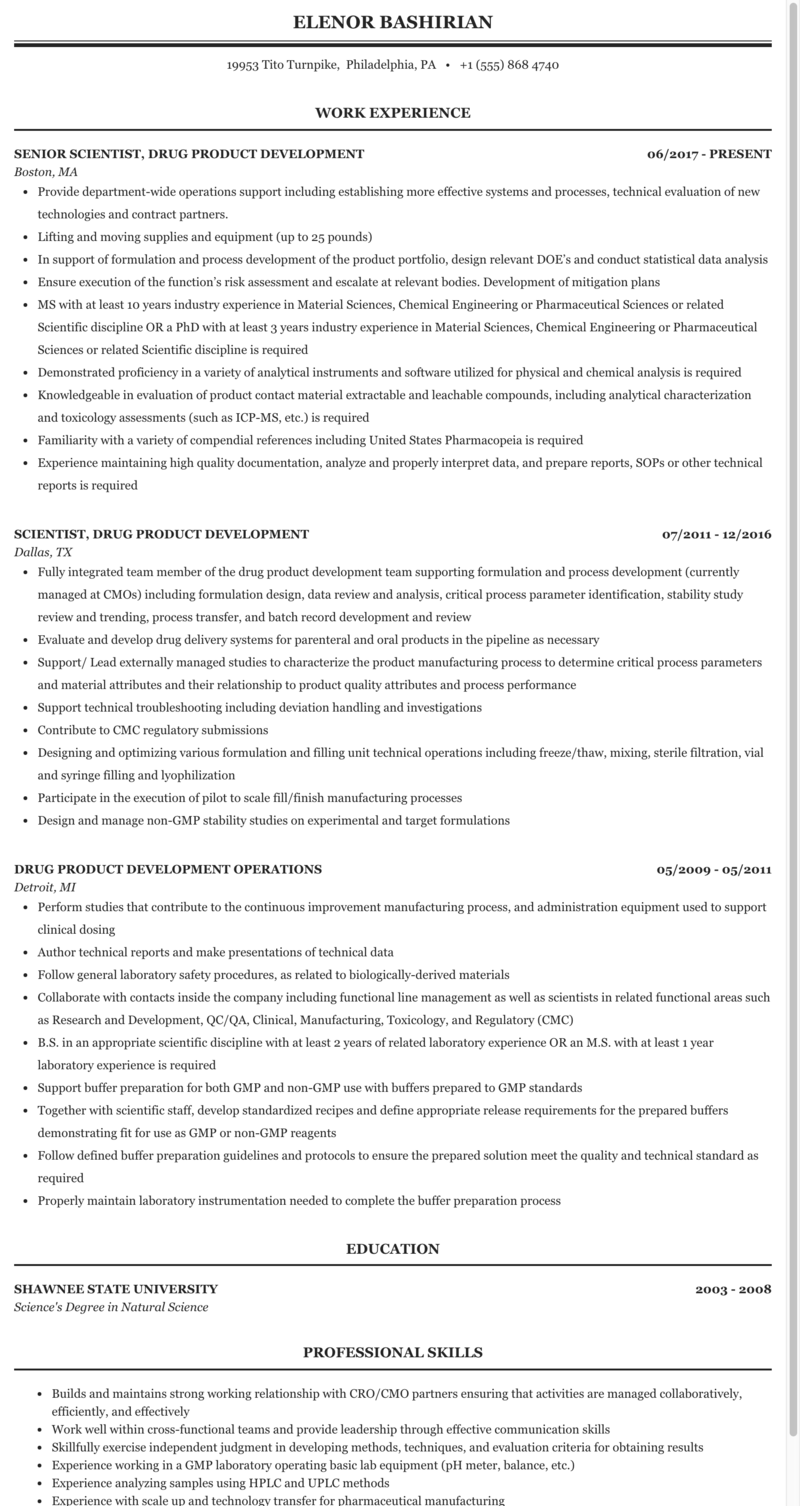
Formulation R&d Resume

Pharmaceutical RD Formulation Developer.
Formulation r&d resume. Opening For Freshers In Denville USA For Quality Control Analytical Formulation RD At Ricon Pharma Jobs October 15 2021 Analytical Research Development Formulation Development Freshers Quality Control USA Jobs. -Contributed to relevant blogs conferences and events both off-line and online to increase brand awareness. Manager Formulation RD is responsible for pharmaceutical product development and associated activities.
RD center of Mascot is having modern Formulation Development FD and Analytical development AD facility which is equipped with modern equipments and instruments. Formulation Scientist Resume Examples Formulation Scientists develop and test mixtures of different compounds to determine which components are needed and in what quantity to produce the desired effect. Pharmaceutical Administration and Drug Regulatory.
Analytical method development validation and transfer. Rdformulation Chemist II Planned and conducted research to generate basic knowledge in semiconductor cleaning and potential new cleaningetching products. Ad Top Resume Builder Build a Perfect Resume with Ease.
Demonstrated ability to work well under pressure multitasking meeting dead lines and full cooperation with colleagues engineers and supervisors. Position summary The main role of the RD Formulation Scientist Consumer Health Products is to research develop and optimize product formulations for various brands within Valeants Consumer Health Care Products portfolio ensuring they. Data Entry Objective Resume Sample Resume formulation rd resume barista resume objective adam resume resolve customer complaints resume stocking skills for resume Learn How Job Search Technology Works and Use it to Your Advantage Technology is.
Aurobindo pharma ltd Walk-in interview 2021 for Formulation Analytical Department f At RD center Bachupally HyderabadAurobindo pharma ltd Notification full detailes belowInterested and eligible candidates Send Your resume. Ad Top Resume Builder Build a Perfect Resume with Ease. Fluent English written and spoken Maximum 5 years of professional experience including residency foundation programme Right to work in Belgium.
This position is responsible for working with cross-functional departments such as Manufacturing QA QC ARD Regulatory Affairs and Portfolio Management. -Designed and created marketing collateral for sales meetings trade shows. Formulation Development Laboratory is supported by Analytical Development Laboratory which carries out the following activities as per GLP requirements.